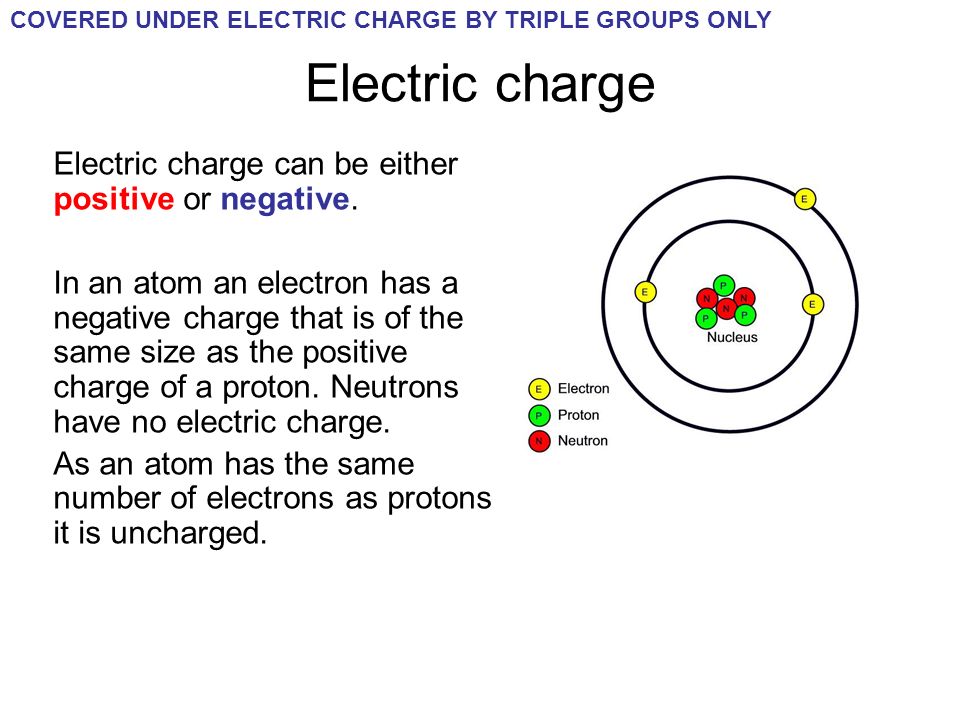
Do Atoms Have An Charge . When they do, they form charged particles called ions: The most common charges are based on maximum stability for the atom. They contain electrical energy too. the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. the structure of atoms. the charge on an atom is related to its valence electrons or oxidation state. An atom consists of a positively charged nucleus surrounded by one or more negatively. atoms can lose or gain electrons. Atoms aren't just packets of matter: how do atoms make ions? If an atom loses one or more electrons, it becomes a positively charged ion. protons are positively charged, while neutrons have no charge. So, the nucleus carries a net positive charge.
from periodictable.me
The most common charges are based on maximum stability for the atom. the charge on an atom is related to its valence electrons or oxidation state. They contain electrical energy too. atoms can lose or gain electrons. the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. If an atom loses one or more electrons, it becomes a positively charged ion. An atom consists of a positively charged nucleus surrounded by one or more negatively. protons are positively charged, while neutrons have no charge. how do atoms make ions? Atoms aren't just packets of matter:
What are the Difference Between Charge and Electron? Dynamic Periodic
Do Atoms Have An Charge They contain electrical energy too. the structure of atoms. Atoms aren't just packets of matter: When they do, they form charged particles called ions: atoms can lose or gain electrons. So, the nucleus carries a net positive charge. protons are positively charged, while neutrons have no charge. An atom consists of a positively charged nucleus surrounded by one or more negatively. They contain electrical energy too. how do atoms make ions? If an atom loses one or more electrons, it becomes a positively charged ion. the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. the charge on an atom is related to its valence electrons or oxidation state. The most common charges are based on maximum stability for the atom.
From telgurus.co.uk
What is the overall charge of the nucleus of an atom and why? Do Atoms Have An Charge atoms can lose or gain electrons. Atoms aren't just packets of matter: the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. So, the nucleus carries a net positive charge. how do atoms make ions? When they do, they form charged particles called ions: An atom consists. Do Atoms Have An Charge.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Do Atoms Have An Charge protons are positively charged, while neutrons have no charge. They contain electrical energy too. the structure of atoms. atoms can lose or gain electrons. The most common charges are based on maximum stability for the atom. If an atom loses one or more electrons, it becomes a positively charged ion. So, the nucleus carries a net positive. Do Atoms Have An Charge.
From www.slideserve.com
PPT Atoms and Their Interactions PowerPoint Presentation, free Do Atoms Have An Charge Atoms aren't just packets of matter: how do atoms make ions? the structure of atoms. the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. The most common charges are based on maximum stability for the atom. protons are positively charged, while neutrons have no charge.. Do Atoms Have An Charge.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Do Atoms Have An Charge atoms can lose or gain electrons. the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Atoms aren't just packets of matter: So, the nucleus carries a net positive charge. The most common charges are based on maximum stability for the atom. protons are positively charged, while. Do Atoms Have An Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Do Atoms Have An Charge If an atom loses one or more electrons, it becomes a positively charged ion. protons are positively charged, while neutrons have no charge. They contain electrical energy too. An atom consists of a positively charged nucleus surrounded by one or more negatively. So, the nucleus carries a net positive charge. the single most important characteristic of an atom. Do Atoms Have An Charge.
From www.numerade.com
SOLVED What types of charge does a proton have? Atomic Structure Do Atoms Have An Charge protons are positively charged, while neutrons have no charge. They contain electrical energy too. the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. the structure of atoms. the charge on an atom is related to its valence electrons or oxidation state. how do atoms. Do Atoms Have An Charge.
From www.slideserve.com
PPT The Atom PowerPoint Presentation, free download ID5056341 Do Atoms Have An Charge atoms can lose or gain electrons. They contain electrical energy too. the charge on an atom is related to its valence electrons or oxidation state. So, the nucleus carries a net positive charge. Atoms aren't just packets of matter: how do atoms make ions? protons are positively charged, while neutrons have no charge. the single. Do Atoms Have An Charge.
From cezqellr.blob.core.windows.net
Do Atoms Have A Electrical Charge at James Ivers blog Do Atoms Have An Charge the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. So, the nucleus carries a net positive charge. the structure of atoms. protons are positively charged, while neutrons have no charge. the charge on an atom is related to its valence electrons or oxidation state. . Do Atoms Have An Charge.
From cezqellr.blob.core.windows.net
Do Atoms Have A Electrical Charge at James Ivers blog Do Atoms Have An Charge how do atoms make ions? The most common charges are based on maximum stability for the atom. protons are positively charged, while neutrons have no charge. the structure of atoms. They contain electrical energy too. If an atom loses one or more electrons, it becomes a positively charged ion. the single most important characteristic of an. Do Atoms Have An Charge.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Do Atoms Have An Charge Atoms aren't just packets of matter: the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. how do atoms make ions? If an atom loses one or more electrons, it becomes a positively charged ion. When they do, they form charged particles called ions: The most common charges. Do Atoms Have An Charge.
From calebcroomphysci4dummies.weebly.com
Atomic Structure Physical Science For Dummies Do Atoms Have An Charge the charge on an atom is related to its valence electrons or oxidation state. When they do, they form charged particles called ions: atoms can lose or gain electrons. An atom consists of a positively charged nucleus surrounded by one or more negatively. The most common charges are based on maximum stability for the atom. the single. Do Atoms Have An Charge.
From cevxgumq.blob.core.windows.net
Does Hydrogen Atoms Have A Charge at Erik Brinkerhoff blog Do Atoms Have An Charge protons are positively charged, while neutrons have no charge. They contain electrical energy too. atoms can lose or gain electrons. the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. the structure of atoms. If an atom loses one or more electrons, it becomes a positively. Do Atoms Have An Charge.
From www.slideserve.com
PPT Atoms and Nuclear Chemistry PowerPoint Presentation, free Do Atoms Have An Charge If an atom loses one or more electrons, it becomes a positively charged ion. The most common charges are based on maximum stability for the atom. the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. protons are positively charged, while neutrons have no charge. They contain electrical. Do Atoms Have An Charge.
From fity.club
What Is The Charge Of An Electron Science Trends Do Atoms Have An Charge The most common charges are based on maximum stability for the atom. the structure of atoms. atoms can lose or gain electrons. If an atom loses one or more electrons, it becomes a positively charged ion. When they do, they form charged particles called ions: protons are positively charged, while neutrons have no charge. how do. Do Atoms Have An Charge.
From studyqueries.com
Charge Of Proton Definition, Mass & More Do Atoms Have An Charge atoms can lose or gain electrons. When they do, they form charged particles called ions: the single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. So, the nucleus carries a net positive charge. how do atoms make ions? protons are positively charged, while neutrons have no. Do Atoms Have An Charge.
From wghsjuniorscience.weebly.com
Atomic structure WGHS Junior Science Do Atoms Have An Charge So, the nucleus carries a net positive charge. the structure of atoms. the charge on an atom is related to its valence electrons or oxidation state. Atoms aren't just packets of matter: The most common charges are based on maximum stability for the atom. the single most important characteristic of an atom is its atomic number (usually. Do Atoms Have An Charge.
From www.youtube.com
What is Electric Charge and How Electricity Works Electronics Basics Do Atoms Have An Charge protons are positively charged, while neutrons have no charge. atoms can lose or gain electrons. If an atom loses one or more electrons, it becomes a positively charged ion. So, the nucleus carries a net positive charge. Atoms aren't just packets of matter: An atom consists of a positively charged nucleus surrounded by one or more negatively. They. Do Atoms Have An Charge.
From cezqellr.blob.core.windows.net
Do Atoms Have A Electrical Charge at James Ivers blog Do Atoms Have An Charge the structure of atoms. protons are positively charged, while neutrons have no charge. The most common charges are based on maximum stability for the atom. Atoms aren't just packets of matter: They contain electrical energy too. When they do, they form charged particles called ions: how do atoms make ions? An atom consists of a positively charged. Do Atoms Have An Charge.